The Bare Symphony of Silence: Minimalism in Epigenetic Inheritance
Gene expression, the grand symphony of life, thrives not just on vibrant crescendos but also on moments of profound hush. It's how silenced genes, like instruments tucked away in their cases, add depth and dimension to the biological concerto. Understanding how this silence is preserved across generations – the realm of epigenetic inheritance – has been a scientific quest with profound implications. A recent study, published in the Proceedings of the National Academy of Sciences (PNAS) titled "Minimal requirements for the epigenetic inheritance of engineered silent chromatin domains," offers a stunning revelation: the score for gene silencing might be simpler than we ever imagined.
The stage for this discovery lies within the enigmatic realm of heterochromatin, a dense genomic fortress where gene expression plays a muted melody.
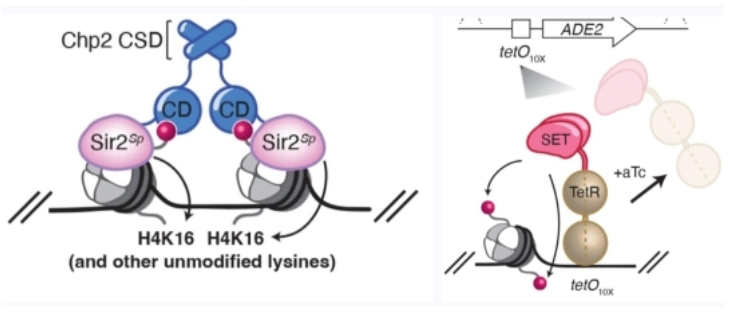
Think of it as a castle guarding specific genes, its walls studded with chemical modifications on histone proteins, the conductors of DNA packaging. The researchers' ingenious twist? Introducing this "silent fortress" (in the form of H3K9 methylation, a histone modification) into Saccharomyces cerevisiae, a humble yeast naturally lacking it. This blank canvas allowed them to study the construction and inheritance of silent chromatin domains from the ground up, unencumbered by pre-existing melodies.
And what emerged was a minimalist masterpiece. Two meticulously designed "bilingual" proteins became the key.
One acts as a translator, reading the silence signal (H3K9 methylation) and writing a silencing mark (deacetylated H4K16) on another histone. The other protein, in turn, reads this silencing mark and writes back the original H3K9 methylation, forming a self-perpetuating loop. This loop amplifies the silence like a whispering echo, ensuring silenced genes remain that way even through cell divisions.
This elegant simplicity debunks traditional models, often cluttered with a multitude of proteins and intricate interactions. Here, just two proteins, dancing a waltz of chemical modifications, orchestrate the inheritance of silence. It suggests a surprisingly lean core mechanism for epigenetic inheritance, with additional layers of complexity added depending on the organism and context.
However, minimalism comes with its own constraints. Building these silent fortresses takes time, requiring a critical density of modifications to solidify the walls. Additionally, maintaining the silence likely relies on other factors, like cellular machinery that retains parental histones during cell division.
While the study lays bare a minimal blueprint for epigenetic inheritance, the story continues. Future research needs to explore how this core mechanism interacts with the intricate cellular orchestra and adapts to diverse scenarios. Perhaps, by mastering the language of silence, we can one day rewrite the expression patterns of disease genes or unlock the secrets of cellular differentiation.
The implications of this research extend far beyond the confines of a yeast lab. Epigenetic inheritance plays a crucial role in development, evolution, and even human diseases like cancer. Understanding its minimalism opens doors to potential therapeutic interventions targeted at specific histone modifications or protein interactions. Imagine silencing silenced genes in cancer cells or reactivating developmentally silenced genes in regenerative medicine.
The PNAS study is a pivotal chord in our understanding of epigenetic inheritance. By revealing a surprisingly minimalist core mechanism, it paints a fresh canvas for gene silencing and paves the way for exciting future research. The orchestra of life can now play with a deeper understanding of its hushed notes, promising advancements in our grasp of the intricate melodies that define biological complexity.
Minimalism on the Epigenetic Stage: How Engineered Silent Chromatin Domains Shake Neo-Darwinism
This study published in PNAS throws down a fascinating gauntlet challenging the Neo-Darwinian paradigm. By constructing a simplified system within the yeast Saccharomyces cerevisiae, the researchers reveal that epigenetic inheritance - the transmission of gene expression patterns without changes in DNA sequence - can be surprisingly minimalistic. This has profound implications for our understanding of evolution and heredity, potentially undermining core tenets of Neo-Darwinism.
Traditionally, Neo-Darwinism posits that natural selection acts solely on heritable variations in DNA sequences. However, the yeast experiment demonstrates that epigenetic patterns can be stably propagated across generations even without any underlying DNA changes. This challenges the centrality of DNA mutations in evolutionary theory, suggesting that inheritance can operate through alternative mechanisms beyond the classic gene-based model.
The researchers' simplified system involves two interlinked proteins that modify histones, the spools around which DNA is wound. These proteins work in a "feedback loop," reinforcing a silent chromatin state where specific genes are suppressed. This epigenetic memory persists through cell division, even though the DNA sequence itself remains unaltered.
This elegant experiment unveils several key points:
Epigenetic inheritance can be remarkably simple: Requiring only two proteins, this system sheds light on the potential ubiquity of such mechanisms in nature. It suggests that complex gene regulatory networks may not always be necessary for epigenetic memory.
DNA mutations may not be the sole drivers of evolution: If epigenetic patterns can evolve and be stably inherited, they become an additional player in the evolutionary game. This could lead to faster adaptation, phenotypic diversity, and potentially even the emergence of non-Darwinian evolutionary modes.
Neo-Darwinism needs refinement: The yeast experiment underscores the need for a more nuanced understanding of inheritance beyond strictly DNA-based mutations. Integrating epigenetic mechanisms is crucial for a comprehensive picture of evolution.
It's important to note that the yeast experiment offers a compelling case for expanding either changing or replacing neo-Darwinism. Epigenetics acts as a complementary player, adding layers of complexity and flexibility to the evolutionary stage. By acknowledging the potential of epigenetic inheritance, we can paint a richer picture of how organisms adapt, diversify, and thrive in a changing world.
In conclusion, the engineered silent chromatin domains in yeast offer a captivating glimpse into the minimalist underpinnings of epigenetic inheritance. This challenges us to move beyond the confines of purely DNA-centric views of evolution and embrace the fascinating interplay between genes, environments, and the epigenetic layer that orchestrates their dance. Only then can we truly appreciate the full spectrum of forces shaping the tapestry of life.





Comments
Post a Comment