Small RNAs in Transgenerational Inheritance of Epigenetics- a challenge to Neo-Darwinism
The 2019 Cell paper, "Small RNAs in the Transgenerational Inheritance of Epigenetic Information," presents a compelling challenge to the neo-Darwinian framework, which has long dominated evolutionary thought. Neo-Darwinism, the modern synthesis of Darwin's theory of evolution and Mendelian genetics, emphasizes random genetic mutations as the primary source of variation, with natural selection acting upon this variation to drive evolutionary change. While acknowledging the role of environmental factors in influencing an individual's phenotype, neo-Darwinism largely relegates such influences to a non-heritable, transient role. The Cell paper, however, suggests a mechanism by which environmental impacts can become heritable, potentially altering the evolutionary trajectory of populations.
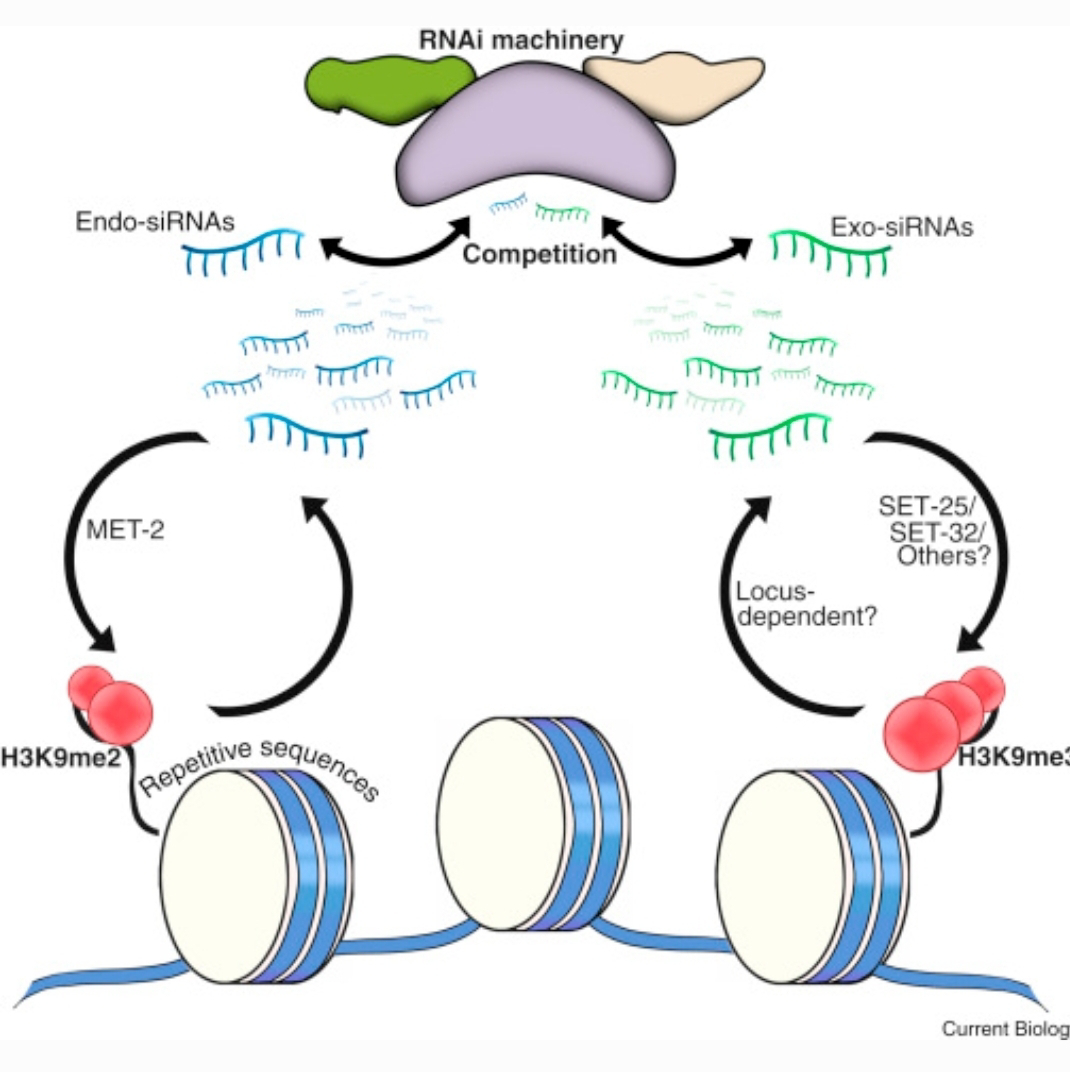
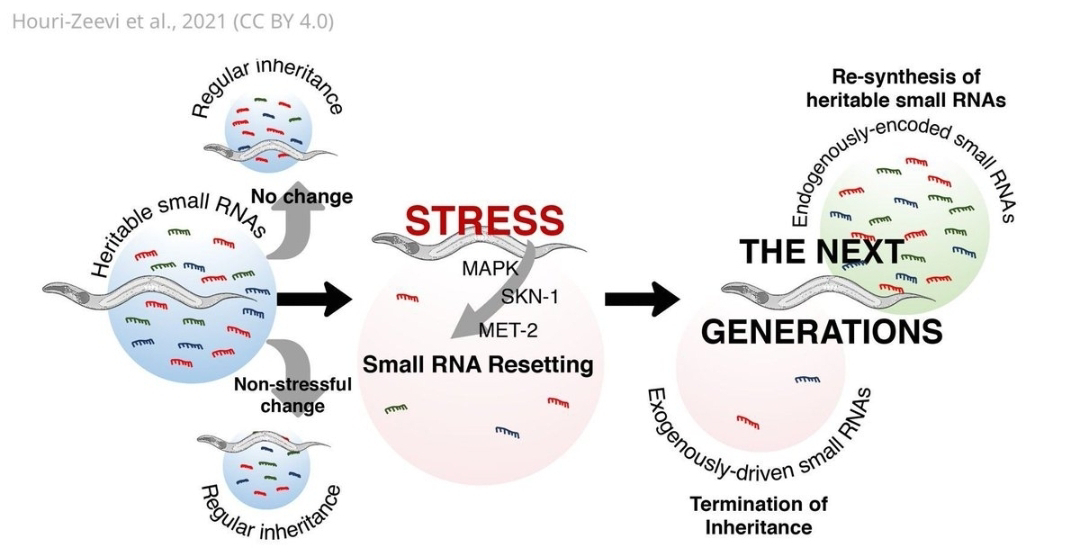
The central players in this challenge are small RNAs, a class of non-coding RNA molecules with diverse regulatory functions within cells. The researchers demonstrated that these small RNAs can be modified in response to environmental cues, such as stress or dietary changes.
Crucially, these modifications are not merely temporary adjustments within an individual; they can be passed down through generations, influencing the gene expression patterns of offspring even when those offspring haven't experienced the original environmental trigger. This transgenerational epigenetic inheritance, mediated by small RNAs, suggests that an organism's experiences can directly shape the biology of its descendants, a concept that sits uneasily within the traditional neo-Darwinian framework.
This discovery has profound implications. It suggests that evolution might be more Lamarckian than previously acknowledged, with acquired characteristics potentially being inherited. While not a complete return to Lamarckism, which posited the inheritance of traits acquired through use or disuse, the findings indicate that environmental pressures can induce heritable changes beyond the DNA sequence itself. This challenges the gene-centric view of inheritance, expanding the concept of heritable information to include epigenetic modifications carried by small RNAs.
The implications extend beyond evolutionary biology. Understanding how environmental factors influence gene expression across generations could revolutionize our approach to human health. For instance, maternal diet or exposure to toxins during pregnancy might have lasting impacts on the health and development of future generations, not just the immediately conceived child. This perspective necessitates a shift towards considering the long-term, transgenerational consequences of environmental exposures. Furthermore, the discovery of small RNAs as carriers of heritable information opens new avenues for therapeutic interventions. If we can understand how to manipulate these epigenetic marks, we might be able to develop treatments for diseases linked to aberrant gene expression, including cancer, diabetes, and neurological disorders.
The precise mechanisms by which small RNAs transmit epigenetic information across generations remain to be fully elucidated. Moreover, the extent to which transgenerational epigenetic inheritance contributes to long-term evolutionary change is still debated. Despite these uncertainties, the paper has undeniably ignited a crucial conversation about the limitations of the neo-Darwinian synthesis and the potential importance of epigenetics in evolution and human health. It underscores the complexity of inheritance and highlights the need for a more integrative approach that considers the interplay between genes, environment, and epigenetic modifications in shaping the characteristics of living organisms. Future research promises to further unravel the intricate roles of small RNAs in mediating transgenerational communication and their contribution to the grand tapestry of life.



Comments
Post a Comment