The Paw-Print of Environment: Epigenetics, Pet Breeding, and the Shifting Landscapes of Inheritance
The burgeoning field of epigenetics is rapidly transforming our understanding of inheritance, with profound implications for how we approach pet breeding. The journal article "Epigenetics in Pet Breeding: Current Insights and Future Perspectives" by Liu, Li, and Xuan (2024) encapsulates this shift, highlighting how heritable changes in gene expression, operating independently of DNA sequence alterations, are increasingly recognized for their role in shaping the traits, health, and diversity of companion animals. This exploration into the epigenetic realm not only offers exciting avenues for enhancing pet well-being but also presents compelling challenges to the traditional framework of neo-Darwinism.
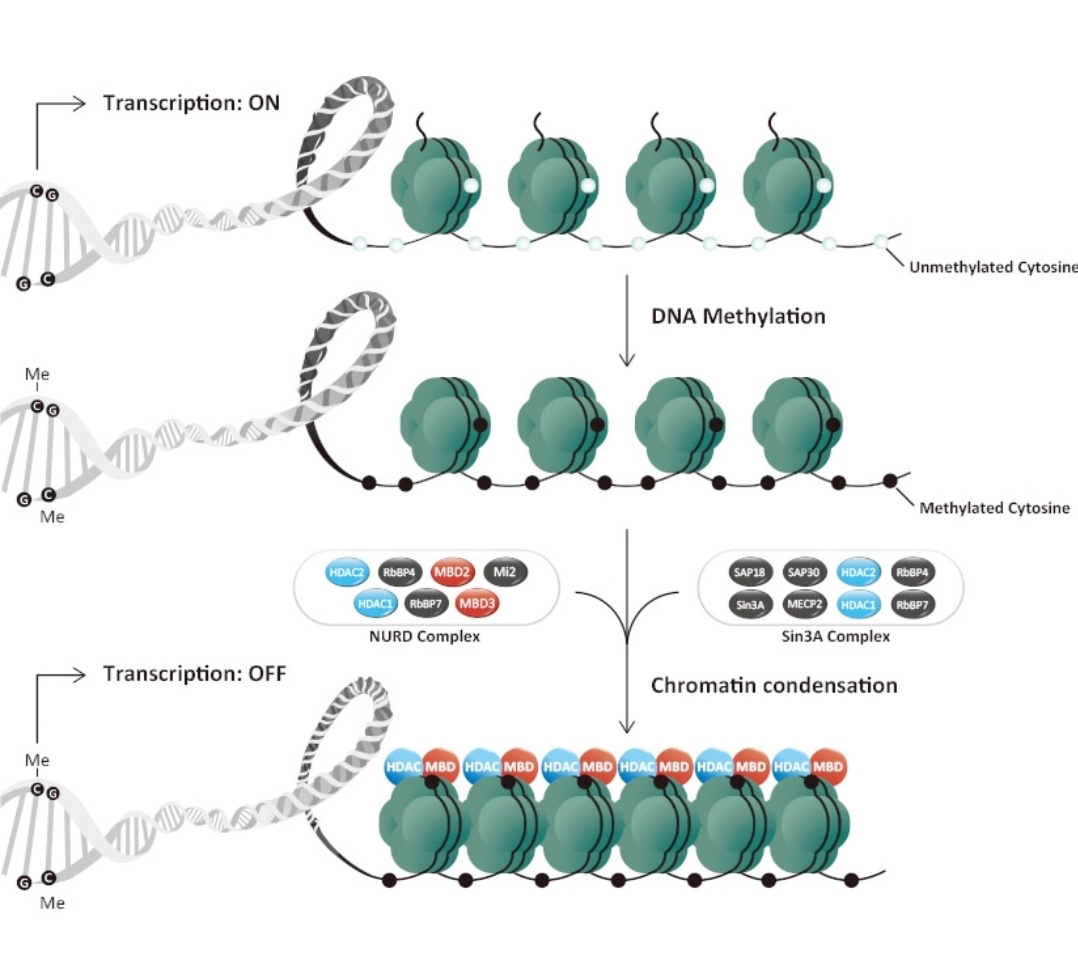
Epigenetics, at its core, involves molecular mechanisms that act as a sophisticated regulatory layer "on top of" the genome.
These mechanisms primarily include DNA methylation, histone modifications, and the activity of non-coding RNAs. As Liu et al. (2024) discuss, these processes can switch genes on or off, or modulate their level of activity, thereby influencing a pet's phenotype – its observable characteristics, from coat color and size to susceptibility to diseases and even behavioral tendencies. For instance, DNA methylation, the addition of a methyl group to a DNA molecule, can silence gene expression.Conversely, modifications to histones, the proteins around which DNA is wound, can alter chromatin structure, making genes more or less accessible for transcription.The critical insight for pet breeding is that these epigenetic marks are not static; they can be dynamically influenced by a wide array of environmental factors throughout an animal's life, and sometimes, these changes can be passed down to subsequent generations. This is where the traditional understanding of inheritance begins to expand. The diet of a mother dog, the stress levels experienced by a queen cat during pregnancy, or even early life socialization and exposure to toxins can potentially leave an epigenetic imprint on their offspring. Research highlighted by sources such as Pupstarts Breeders and Dogs Naturally Magazine emphasizes that the uterine environment and early post-natal experiences are critical windows for epigenetic programming, impacting everything from temperament to adult health. Studies on DNA methylation in canine brains, for example, suggest that epigenetic factors have played a significant role in the divergence of dogs from wolves and in the formation of distinct breed characteristics, particularly in relation to behavior and morphology.
This notion of heritable environmental influence directly challenges certain tenets of neo-Darwinism. The Modern Synthesis, or neo-Darwinism, traditionally posits that evolution occurs through the gradual accumulation of random genetic mutations, with natural (or artificial, in the case of breeding) selection acting upon the resulting phenotypic variation. The primary vehicle of inheritance is considered to be the DNA sequence itself. Acquired characteristics, meaning traits developed during an organism's lifetime due to environmental influences, are generally not considered heritable under this framework, a concept that historically distanced Darwinian evolution from Lamarckian ideas.
Epigenetics, however, introduces a mechanism for what can be described as a "soft" inheritance or a neo-Lamarckian influence. If environmental factors can induce epigenetic changes that alter an animal's traits, and if these epigenetic marks can, even partially, escape the typical reprogramming events during gamete formation and early embryogenesis to be transmitted to the next generation, then the environment is playing a more direct role in shaping heritable variation than previously acknowledged by strict neo-Darwinian views.
The article by Liu et al. (2024) points towards the "potential to influence breed characteristics, health, and genetic diversity" through understanding these epigenetic mechanisms. This implies that breeders might, in the future, consider the environmental management of parent animals not just for their immediate well-being, but for the lasting epigenetic legacy they impart on their litters. For example, specific dietary regimens for breeding pairs or stress-reduction protocols could become integral to producing healthier, more resilient offspring with desired temperaments.
The challenge to neo-Darwinism lies in several key areas:
Rapidity of Change: Epigenetic modifications can occur much more rapidly than the accumulation of advantageous DNA mutations. This could explain instances of rapid adaptation or alteration of traits in domesticated species, such as the famous Russian fox experiment, where foxes selected for tameness exhibited significant behavioral and physical changes (like floppier ears and mottled fur, potentially linked to methylation of genes like Agouti) within a remarkably small number of generations. These changes often preceded corresponding genetic alterations.
Directed Variation: While genetic mutations are considered random, some epigenetic changes can be a more direct response to specific environmental cues. This suggests a pathway for the environment to induce adaptive phenotypic changes that can then be inherited, providing a more targeted response than purely random mutation.
Inheritance of Acquired Traits: The core of the challenge. If, for example, a dog's predisposition to anxiety is heightened due to epigenetic modifications triggered by its parent's stressful experiences, and this predisposition is passed to its puppies, it represents a form of acquired trait inheritance. While the extent and stability of transgenerational epigenetic inheritance in mammals are still subjects of intense research and debate – with many epigenetic marks being reset between generations – evidence is mounting that some marks do persist.
Epigenetics challenges neo-Darwinian mechanisms as it extends and enriches our understanding of evolution and inheritance. As some researchers propose, environmental epigenetics might provide molecular mechanisms that can facilitate neo-Darwinian evolution by directly altering phenotypic variation upon which selection can act. Epigenetic changes might also influence genetic stability and mutation rates, further intertwining these two inheritance systems.
The future perspectives outlined by Liu et al. (2024), such as "personalized breeding, informed by epigenetic data," suggest a paradigm where breeders are not only selecting for desirable genes but are also actively managing the epigenomes of their animals. This could involve epigenetic screening, tailored diets, and optimized environments to promote beneficial epigenetic marks and mitigate detrimental ones.
In conclusion, the study of epigenetics, as highlighted in "Epigenetics in Pet Breeding: Current Insights and Future Perspectives," is opening a new chapter in our approach to animal husbandry. It reveals that the environment writes on the slate of heredity in ways more intricate and immediate than previously understood. By providing mechanisms for the inheritance of environmentally influenced traits, epigenetics presents a nuanced challenge to the gene-centric view of neo-Darwinism, suggesting that the evolutionary narrative is a dynamic interplay between the enduring script of DNA and the responsive edits of the epigenome. For pet breeders, this means that the care and conditions provided to animals today may sculpt the very nature of the companions of tomorrow in ways we are only beginning to appreciate.





Comments
Post a Comment